Removing flammable gas from water

The model is entirely based on the patent of Hillary Eldridge, USA
603,058 "Electrical Retort" introduced April 26, 1898.
Combustible gas is produced by an electric arc produced
graphite rods immersed in distilled, potable, salted or
another type of water that is essentially made up of hydrogen, oxygen, carbon and
other substances.
The generator produces a mixture of carbon monoxide and hydrogen (COH2),
which burns very cleanly with oxygen in the air, and can be used as
fuel for an internal combustion engine. When burned, COH2 is formed
carbon dioxide and water vapor, so environmental pollution is extremely
insignificant.
Gas analysis by NASA: Hydrogen 46.483%
Carbon dioxide 9.329
Ethylene 0.049
Ethane 0.005
Acetylene 0.616
Oxygen 1.164
Nitrogen 3.818
Methane 0.181
Carbon monoxide 38.370
Total 100.015

This simple experiment is intended solely for
proof of the main concept. This generator cannot be used for
long-term use and is for display purposes only.
You will need few materials, the generator is very simple to build and test....
Be careful, the generator produces explosive gas, You
are required to conduct this experiment in a well-ventilated area or in the open
air. You should not smoke during the experience. Don't forget that carbon monoxide
(CO) - very poisonous, do not inhale it! The experiment is intended only for
experienced The experimenter must be very careful during the experiment!Experiments
are carried out by you at your own peril and risk. I don't take any responsibility
responsibility for anything that may happen due to improper use
this information.
You only need:
- A small plastic bottle of sparkling water,
- two graphite rods (70 mm length, 6 mm diameter)
- one 1 ohm 50Watts resistor
- A DC transformer that can
provide 35v/10A
- wires, connectors and silicon cement, or any other
waterproof composition.

very few materials.....
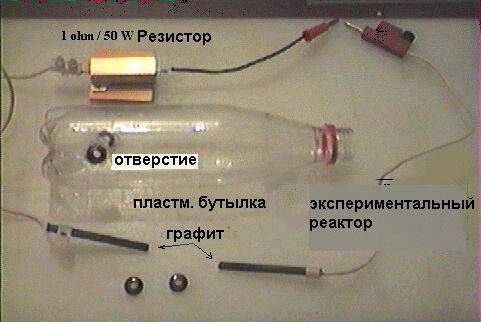
1) Drill two diametrical holes (10 mm diameter) 60 mm from the base of the bottle and
insert graphite rods with (rubber bands from a washing machine - for
sealing) and glue the rubber bands with silicon cement. It is advisable to end
one of the graphite rods was conical. Two rods must be in front
switching on in weak contact (see below).

Connect a 1 ohm 50W resistor in series with one of the graphite rods and
one pole of the transformer (34V/15A DC), the other pole
connect the transformer to another graphite rod. Can add
additional instruments to measure current and voltage. Fill your generator
only with distilled or purified fresh water.

Now you are ready to get flammable gas...















