Burnishing, copper plating at home and where it may come in handy
To effectively protect steel surfaces from corrosion, bluing, passivation and copper plating are used. Such coatings are highly resistant to abrasion, which is superior to paint. You can carry out bluing, passivation or copper plating of steel surfaces at home using inexpensive commercially available materials.
Burnishing with linseed oil
This method involves creating a black protective and decorative oxide film on a steel surface by immersing a part heated to a temperature of 450-470 degrees Celsius in linseed oil. Heating to this level is safe, as it does not interfere with the hardening and hardening of the steel.
The part for bluing is evenly heated with a gas burner; temperature control is carried out with an infrared pyrometer or visually by tarnish colors. When using the latter method, it is necessary to take into account that the correspondence of the tarnish color to a certain temperature is different for ordinary and chromium steel.
When working with ordinary steel, the part is heated until it changes from a gray tarnish to a glow.As the temperature rises, the surface will initially be dark blue, then light blue, then gray. As soon as the gray color turns brown, which happens before the glow begins, the part is immersed in linseed oil. For better effect, the procedure can be repeated 2-3 times.
Once the part has cooled for a couple of seconds, it is removed from the bath and left until it oxidizes. Excess oil will drain off, and the remaining thin layer will form a black oxide film after some time.
Passivation with phosphoric acid
This method of protection involves transferring the thinnest top layer of steel to a neutral state that prevents corrosion. To do this, the cleaned part is immersed for 1 hour in a rust converter, which is a solution of orthophosphoric acid.
As a result, a gray oxide film will appear on the surface. In order for it to be uniform, the part must be thoroughly cleaned and degreased before an acid bath.
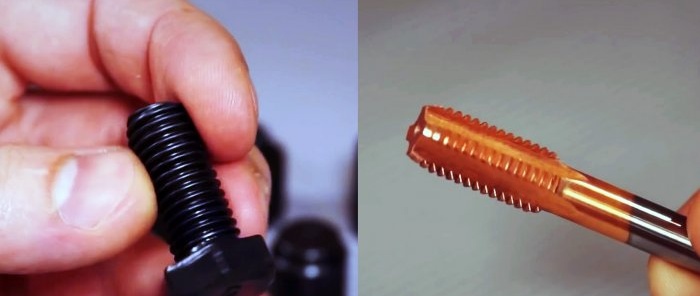
Copper plating with copper sulfate and electrolyte
For copper plating, it is necessary to prepare a solution consisting of 100 g in a plastic or glass container. copper sulfate, 450 gr. distilled water and 100 gr. electrolyte for batteries. The resulting reagent has an unlimited shelf life.
The part for copper plating is cleaned, degreased in a solvent, dried and dipped into the solution. In a matter of moments, a copper coating appears on the steel surface.
Taps can be coated using this method to protect against corrosion and reduce friction when cutting threads. Copper plating will also help to thicken the loose seat under the bearing. It is dipped into the solution several times to increase the diameter using copper until the bearing fits tightly. Copper adheres evenly, so this restoration method will not close the grooves.
Watch the video
Similar master classes
Particularly interesting
Comments (2)