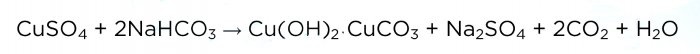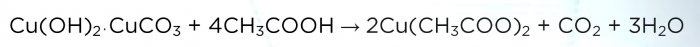Copper acetate crystals at home
Good afternoon Surely you have heard about growing crystals at home from various kinds of reagents: iron or copper sulfate, table salt, citric acid. The color of the crystal depends on the reagent. This time we will be growing beautiful black crystals from copper acetate.
But first, let's get the copper acetate itself (and our crystals will grow in the resulting solution).
Will need
So, we will need:
- Copper sulfate is a fertilizer sold in garden stores;
- Baking soda and vinegar 9% - can be found at the grocery store;
- Distilled water - in a hardware store.
We will also need disposable tableware: cups, spoons, and several filters: coffee or, preferably, chemical.
Obtaining crystals from copper acetate
First, prepare a solution of copper sulfate. Pour vitriol into the bottom of the glass:And fill with distilled water half at a time. Stir until the substance is completely dissolved.
Now add soda in small portions (heaps on the edge of a spoon).Immediately after adding, you should cover the glass with a napkin, because as a result of the reaction, bubbles of carbon dioxide are released, which carry particles of the solution with them. The reaction equation is shown below.
And this is how the solution “hisses”: When carbon dioxide stops being released, you should stop adding soda and let the solution sit for a while. As a result, a transparent layer of solution forms on top:Then we pour the liquid into another glass (we don’t need it) and rinse the precipitate of the main copper carbonate. To do this, add a little distilled water to it, mix, let it settle and drain the liquid. This procedure must be repeated two or three times.
Next, add distilled water to the substance again and pass the solution through a filter: we need to collect the precipitate.
Once we have filtered the precipitate, it needs to be washed a couple of times on the filter. To do this, add water and mix lightly.
After that, put the sediment directly with the filter on a napkin so that it dries a little.
When the sediment dries, transfer it to a new glass. Next we need vinegar. Add it to the solution in small portions until all the sediment has dissolved.
Reaction equation:
The solution turns dark blue.
The precipitate, like copper acetate, has a rather low solubility, which is why it does not dissolve very well when vinegar is added. When the precipitate dissolves, filter the solution.
The filtered solution has a beautiful dark blue color.
Leave the resulting solution for a week. During this time, small crystals will begin to form in it.
After a week, pour the solution into another glass, pour the crystals at the bottom onto a napkin.
We choose the one we like best and tie it to the fishing line. Place it in the solution to grow. Now all that remains is to wait.Every week the solution should be poured into a new glass so that the crystal grows at maximum speed.
During growth, the fishing line also becomes overgrown with crystals, which should be disposed of. Just do this with great care so as not to damage the main crystal.
This concludes my article. Good luck with your repetition everyone!