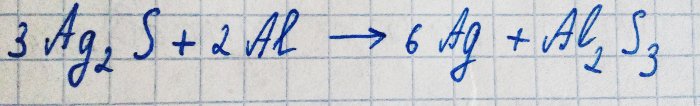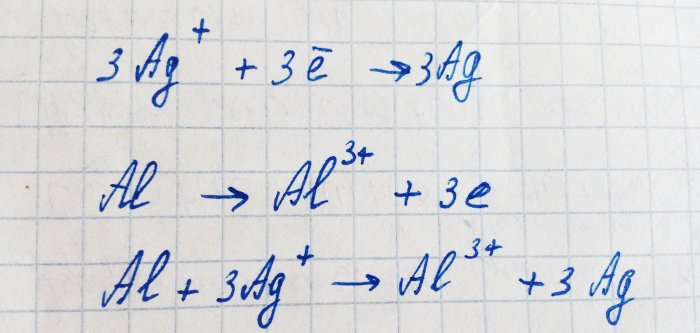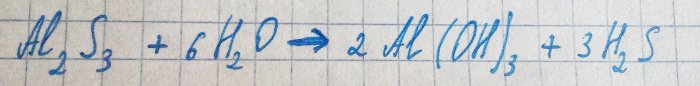Cleaning silver items at home
Recently I came across a set of silver cutlery that is about a hundred years old. During this time, the spoons and knives managed to darken and rust, and I decided to clean them of the deposits that had formed. In this article, I will share a fairly effective way to restore your antique cutlery to its original shine. So, let's go.
Theory
First, let's figure out why silver darkens over time and where rust comes from.
Silver (Ag) exhibits virtually no reactivity with oxygen or water, that is, it does not rust. This property was first noticed by alchemists; they began to call silver a noble metal.
However, silver reacts with hydrogen sulfide in the presence of oxygen. The equation for this reaction is as follows:
The resulting silver sulfide just covers the metal with a dark film, preventing further oxidation.
If the cutlery is not made of pure silver (for example, 800 standard), then impurities in the alloy rust.
Rust removal
Prepare a solution of citric acid. The more saturated it is, the faster and more violent the reaction will proceed.We put cutlery in it, when gas bubbles stop emitting, we take them out of the solution, rinse with water and wipe with a paper towel.
For best results, you can heat the solution to 80°C, but it is important to follow safety precautions.
Plaque removal
To remove plaque, we will use the chemical method of reducing silver sulfide to free metal.
For this we need:
- - the container where the reaction will take place;
- - baking soda;
- - aluminium foil;
- - table salt;
- - hot water.
Place aluminum foil on the bottom of the vessel and silver objects on top, dark side down.
Sprinkle soda on top.
Now dissolve the salt in water at the rate of 6 tablespoons per liter of water. Pour the resulting solution into the container, a reaction begins with the release of hydrogen sulfide and carbon dioxide.
The entire process must be carried out outdoors, because hydrogen sulfide has a characteristic smell of rotten eggs and is harmful to health.
Let's look at the chemistry of what is happening.
First, the soda dissolves the thin hydroxide film from the surface of the foil.
This gives the silver sulfide the opportunity to react directly with the aluminum.
Aluminum has a lower ionization energy than silver. It oxidizes to an ion, giving silver its electrons. This process can be written like this:
This reaction is electrochemical: due to the movement of electrons from aluminum to silver, a potential difference appears.
Salt in a solution improves its conductivity, allowing the reaction to proceed to completion.
The released aluminum sulfide immediately reacts with water:
Hence the hydrogen sulfide and the corresponding smell.
This process can take up to half an hour. When finished, rinse the cutlery with water and wipe dry with a towel.
After silver restoration, you can use special means to preserve it. They protect the silver surface from moisture and hydrogen sulfide.
This way, the silver will retain its shine for a long time.
Conclusion
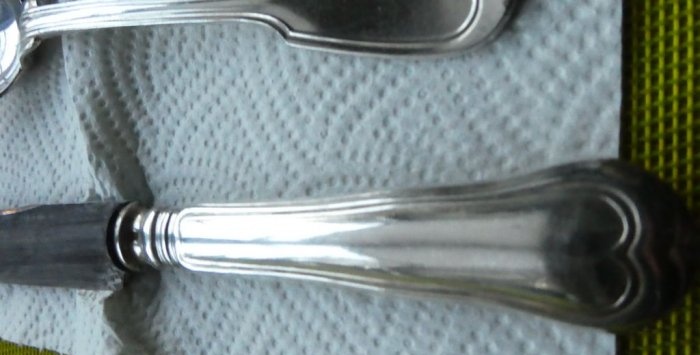
Photos of restored dishes:
As you can see, there is no trace of darkening on the spoons. The knives also began to look better, however, unfortunately, part of the silver layer disappeared from their surface; time still takes its toll. You'll have to take them to a specialist to re-silver them.
And this article has come to an end. Good luck with your repetition everyone!
Original article in English
Similar master classes
Particularly interesting
Comments (0)