Parts restoration: rust converter against soda hydrolysis
You can often get your hands on rusty tools or other metal objects that would be nice to restore. If there is shallow surface corrosion, then after treatment to remove rusty scale, it is quite possible to use the updated parts for their intended purpose. Let's compare hydrolysis technology and dissolving rust with chemical compounds to understand which is better.
Materials
When treating rusty objects by hydrolysis you will need:
- aluminum or copper wire;
- caustic soda;
- water;
- power supply or charger for a car battery;
- open container of suitable size;
- an unnecessary steel object approximately corresponding in size to the part being restored;
- metal brush.
For chemical treatment you will need to prepare:
- "Evapo-Rust" or other rust converter;
- open container of suitable volume;
- latex gloves.
Reduction by hydrolysis
Restoring a part by hydrolysis involves transferring rust from the item being restored to an unnecessary steel workpiece under the influence of electricity. To do this, prepare a concentrated solution of caustic soda.
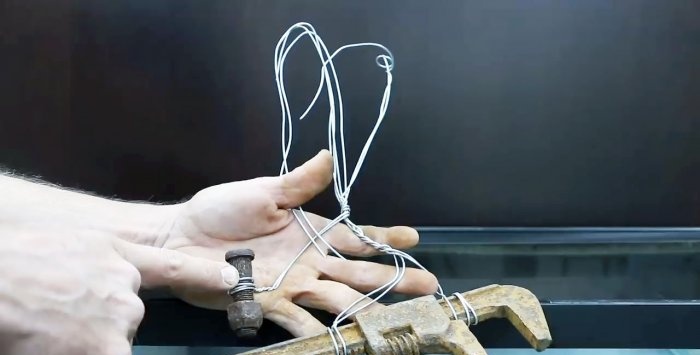
The part to be restored is tightly tied with several strands of aluminum or copper wire so as to leave a long end.
The same procedure is done with the workpiece for receiving rust. Both parts are lowered into a bath of caustic soda solution. There should be a small distance between them. The positive wire from the charger is connected to the wire of the unnecessary workpiece protruding from the bath, and the negative wire to the part being restored.
After this, you need to turn on the charger and first set the minimum voltage.
When power is applied, carbon dioxide bubbles will begin to separate from the workpieces. This is completely safe and normal. At first, you need to observe the operation of the rectifier; if it or the solution does not heat up, then you can add voltage. This will speed up the rust removal process. It is imperative that the charger wires are not immersed in the solution, since their clamps may oxidize. Periodically you will need to tighten the wire on the parts to maintain good contact.
Chemical restoration
The process of restoring parts using chemical compounds is much simpler. You can use a rust converter (Evapo-Rust) or its equivalent. A chemical composition is poured into any container of suitable volume, after which the part requiring restoration is immersed.
It remains in the bathroom for several hours, sometimes a day, depending on the degree of corrosion.
Comparison of methods
Both methods of restoring rusty parts are effective, but objectively using chemical compounds is better.
They remove rust more thoroughly and evenly, and after treatment they do not require mechanical cleaning. In the case of hydrolysis, with a similar processing time, you will still have to use a metal brush at the end.
The duration of hydrolysis, depending on the power of the charger, the concentration of soda in the water, the weight of the workpiece and the degree of its coverage with rust, can last from several hours to a couple of days. The undoubted advantage of hydrolysis is the low cost of this method.
Both restoration methods, if the process is stopped in a timely manner, will not lead to corrosion of the living metal under the scale.
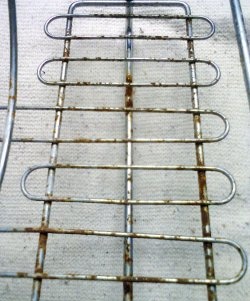
Initially rusty parts and after restoration by hydrolysis with soda:
Now let’s compare rust converter and hydrolysis:
Watch the video
Similar master classes
Particularly interesting
Comments (0)