How to protect a copper soldering iron tip
Before protecting the copper soldering iron tip, it must be tidied up and tinned. As you know, the purer the metal, the slower it oxidizes. The manufacturer decides for himself what purity of copper he used, and we cannot influence this.
The next method of protection is associated with forging, with which you can fix surface cracks, small dents, remove scale, crush large crystals in the metal structure, and increase its hardness. As a result, the metal's resistance to corrosion increases noticeably. This treatment is necessary in cases where the soldering iron is used constantly and intensively.
2 “folk” methods of protection: graphite and aluminum
Let's now deal with protecting the surface of the soldering iron tip. In theory, polished metal resists oxidation better, but only at the initial stage and under non-aggressive corrosive conditions. Therefore, we polish the metal of the tip with a polishing disc. For comparison, part of the surface will be polished by treating it with sandpaper.
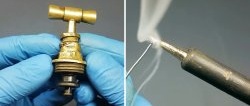
All that remains is to test the effectiveness of the thin protective layer. The most available materials are graphite and aluminum.The process of applying them is quite simple. We rub the surface of the metal tip with a pencil lead or a piece of aluminum wire.
Please note that this method is only compatible with a sanded surface. When polished, graphite leaves no marks, and aluminum adheres only after the polished surface is scratched.
Next, for the convenience and purity of the experiment, we will build a simple stand for a soldering iron from wire, bending it with pliers, and using a square-shaped paper sheet of given dimensions as a meter and template.
We place the soldering iron on the stand, plug it into the network and wait to see what happens. External changes become noticeable within 10-15 minutes. Simulating long-term operation through repeated heating and cooling cycles gives results.
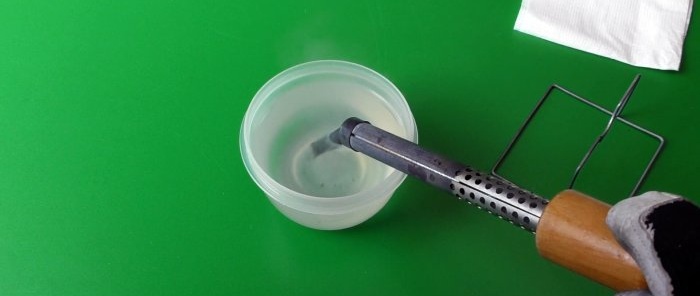
The area where the graphite was applied is not particularly different from the uncoated surface. That is, graphite protection is not very reliable. But the area rubbed with aluminum is very clearly visible, and the metal underneath has not oxidized. To complete the experiment, let’s lower the hot tip into the water, but do it slowly and gradually so as not to get burned by the splashes of boiling water.
We dry the tip with a paper napkin and under the layer of scale we observe the following. Aluminum showed the best result. But aluminum processing is more relevant for soldering irons with a thin tip. If the tip is thick, then rubbing in the aluminum becomes a labor-intensive process, but it's worth it.