Experiment: how to coat a part with copper, nickel, brass and aluminum at home using electrolysis
Copper, nickel, brass and aluminum are resistant to corrosion, so a thin layer of them on the surface of the steel can protect it from rust. One metal can be deposited onto another using electrolysis. But it doesn't always work. Let's test it on the proposed metals.
What you will need:
- metal samples;
- vinegar;
- salt;
- DC power supply;
- plastic containers.
Electrolysis process for copper, nickel, brass and aluminum
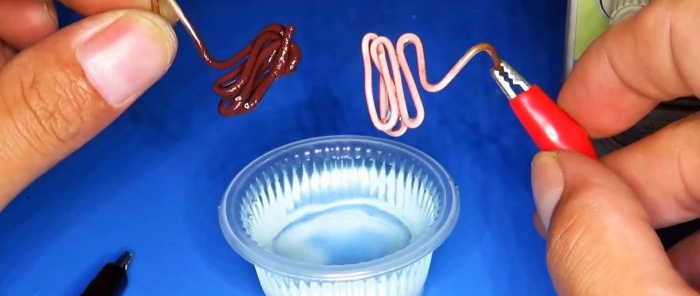
For electrolysis it is necessary to prepare an electrolyte. Vinegar is used as it. The process is performed in a plastic container, since it is a dielectric. Salt is added to vinegar for better conductivity.
For copper plating, you need to bend 2 electrodes from copper wire, lower them into the electrolyte and connect the wires to the power supply.
After 20 minutes, the electrode at the positive terminal will be cleared of oxide, which will move to the negative terminal.
Now if you connect a steel object to the negative wire, it will be covered with a uniform, neat layer of copper.
For nickel plating, a similar action is repeated with two electrodes made of this metal. After 20 minutes, a steel part clings to the negative wire. It will also be coated with a layer of nickel.
If you repeat the experiment with brass, then nothing will work. Only oxide will appear on the steel part. It won't look like brass.
Transferring aluminum to steel does not work either. During electrolysis, the electrolyte will only become contaminated and turn dark gray. The detail itself will generally remain unchanged.