An affordable, simple way to chrome plating metal at home
A shiny layer of chrome on metal children and tools makes them beautiful and also reliably protects them from corrosion. Its application is possible not only in production, but also at home using available equipment. A coating made in a workshop in this way is highly resistant to abrasion, so it is advisable to apply it even on surfaces that are subject to intensive use.
What you will need:
- Stainless steel;
- table vinegar 9%;
- table salt;
- powder cleaner;
- DC source.
The process of chrome plating parts at home
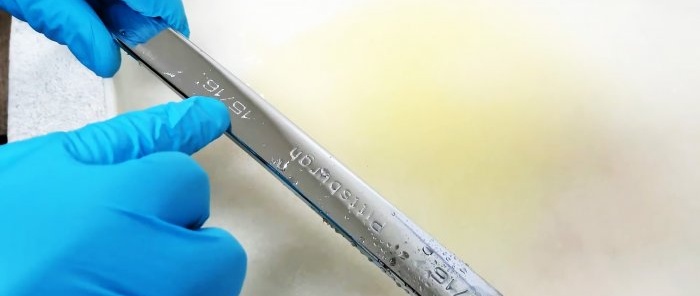
To perform chrome plating, you will need to make 2 electrodes from stainless steel (more precisely, from ferritic-chromium stainless steel, otherwise not everyone will understand where the chrome came from), which is not attracted by a magnet. It contains a high enough amount of chromium to be extracted and used for coating. Old spoons are ideal for this.In this case, one such spoon was found (it uses inexpensive ferritic-chromium stainless steel), and a strip of stainless steel was cut out for the second electrode.
For easy connection of wires, holes are drilled in the electrodes.
Vinegar is poured into a glass container in such a volume as to completely cover the part for chrome plating. Table salt is poured on top at the rate of a handful per 0.5 liter of acetic acid.
Electrodes with connected wires from a direct current source are lowered into the container. This could be a power bank, a transformer, a charger for car batteries. To prevent short-circuiting, a dielectric separating insert, such as a piece of plastic food container or bottle, is placed between the electrodes.
Turn on the power and wait until the electrolyte is saturated and turns black.
After this, it must be filtered through cotton wool.
The part for chrome plating should be cleaned before processing. Any scratches or other defects on it will be visible through the chrome layer, so if necessary, it must literally be polished.
The workpiece is then thoroughly washed in a powder cleaning solution. It will degrease the surface and allow chrome to be attached to it.
The electrolyte in a glass container must be heated to a temperature of 60-95 degrees Celsius, for example, by placing an electric stove. Then a higher quality stainless steel electrode, in this case a spoon, and a part for chrome plating are dipped into it. The minus from the power source is connected to the electrode, and the plus to the workpiece. The speed of chrome plating depends on the power of the transformer or charger used.
The result is a durable, shiny finish. It fits especially well on brass and copper-plated surfaces.If the part is made of steel, and in the future it will be used in difficult conditions, then it is better to first carry out copper plating and then chrome plating.
Watch the video
Galvanizing steel at home - https://home.washerhouse.com/en/7523-ocinkovka-stali-v-domashnih-uslovijah.html